
Fall 2023
You’re Invited! Advocacy Events at the Annual Meeting
AMP’s 2023 Annual Meeting will be held in Salt Lake City, Utah, from Tuesday, November 14th to Saturday, November 18th. AMP is happy to announce that both AMP’s Professional Relations Committee (PRC) and Economic Affairs Committee (EAC) will be hosting sessions during the Annual Meeting.
The PRC coordinates, strategizes, and implements the Association’s initiatives with the federal government, stakeholder coalitions, trade associations, and patient and professional organizations to inform and influence policy discussions that have an impact on the practice of molecular pathology.
The EAC addresses, advises, and educates payors, legislators, and the public on economic issues of importance to the field of molecular pathology, and develops and advocates for sound economic policies that promote the availability to patients of high quality molecular pathology services. AMP develops its policy positions based on the needs of its membership and in order to ensure they are appropriate and up to date, it is important to connect with the membership and understand their current practice, priorities, and concerns.
On Thursday, November 16th from 10:15-11:45am MST the Economic Affairs Committee (EAC) invites you to attend a discussion on the challenges of delivering patient care in this new economic landscape, with a focus on the Protecting Access to Medicare Act (PAMA), New Current Procedural Terminology (CPT) Codes and Prior Authorization issues. The session will explore the impacts and future implications of PAMA, educate members on new CPT codes that will be implemented for CY2024, and discuss challenges related to the implementation of prior authorization in the laboratory setting. Economic Challenges to Delivering Molecular Diagnostics for Patients at 10:15am.
On Friday, November 17th from 1:15-2:45pm MST the The Professional Relations Committee (PRC) invites you to a two part session celebrating PRC Advocacy successes including the celebration of the 10th anniversary of the historic AMP v. Myriad Supreme Court decision featuring AMP leadership and the CEO of Myriad Genetics. The latter half of the will highlight ongoing work, including the current status of the AMP v. Myriad decision, the recent FDA proposed rule for Laboratory Developed testing Procedures, and AMP’s CLIA Modernization Proposal. This session is an opportunity for attendees to celebrate AMP’s past achievements, and engage with the PRC to learn about current advocacy activities, and how you can become involved in our efforts. AMP Advocacy: Celebrating the Past and Looking Towards the Future at 1:15pm.
AMP Continues Advocates for Appropriate Oversight of Laboratory Developed Testing Procedures (LDPs)
In June 2023, President Biden’s unified regulatory agenda was released and indicated that the FDA would release a draft rule in August claiming oversight and authority for the regulation of Laboratory Developed Tests (LDTs) as Medical Devices. The proposed rule was set to be released in August, but was not. In the rulemaking process, before a proposed rule is published in the Federal Register for public comment, the President, as head of Executive Branch, may take the opportunity to review the rule assisted by the White House Office of Information and Regulatory Affairs (OIRA). OIRA, housed in the Office of Management and Budget (OMB) analyzes draft proposed rules, such as this one, when they are “significant” due to economic effects or because they raise important policy issues. For significant rules, the agency must estimate the costs and benefits of the rule and consider alternate solutions.
During the White House review, AMP met with OIRA regarding the LDT Proposed Rule on August 10th 2023. Dr. Eric Konnick, PRC Chair, presented our concerns with FDA regulation and oversight of Laboratory Developed testing Procedures (LDPs). The purpose of the meeting was to convey AMP’s concerns with regulating LDPs as medical devices, and request that prior to issuing a Notice of Proposed Rulemaking (NPRM), the Administration consider issuing a request for information (RFI) to collect data and better understand the impact of rulemaking on academic medical center laboratories and other clinical laboratories offering localized care. Dr. Konnick stressed that FDA oversight of LDPs would impede patient access to critical testing and stifle innovation. OIRA requested additional information and potential RFI questions from AMP, which were submitted. On September 29th, FDA's proposed rule on LDP regulation was released. The rule was published in the Federal Register on October 3rd, 2023, with an open comment period for 60 days, with a deadline of December 4th. AMP is meeting with various stakeholders regularly and drafting comments. Additionally, AMP is finalizing the CLIA modernization proposal as an alternative legislative pathway for LDP regulation to remain under CMS authority.

AMP Reaffirms Position that Genes Should Not Be Patentable in Response to Newly Introduced Legislation
A defining moment in AMP’s history was the 2013 unanimous Supreme Court decision, AMP v. Myriad, which recognized that DNA is a “product of nature” and therefore, could not be patented. On June 13th, 2023, AMP and advocates for Precision Medicine celebrated the tenth anniversary of the Supreme Court decision Association for Molecular Pathology v. Myriad Genetics, Inc. This ruling upheld the rights of patients, by declaring human genes were not patent eligible, which forever changed the genetic testing landscape to ensure physicians could continue to accurately diagnose and inform treatments for patient conditions. AMP and other stakeholders promoted the landmark decision on social media and through the press which included launching a new advocacy website highlighting the history of the case, as well as a blog post written by Dr. Eric Konnick. Furthermore, AMP presented its Champion of Innovation Award to the Department of Health and Human Services (HHS) Secretary Xavier Becerra for his 30 years of leadership in healthcare reforms that include protecting patient access to critical medical testing procedures.
AMP continues to engage external stakeholders and congressional offices to discuss the harms gene patents will have on patient access to care and the health care system at large.
On June 7th, 2023, AMP led Congressional meetings with Facing Hereditary Cancer Empowered (FORCE), American Civil Liberties Union (ACLU), Invitae, and American College of Medical Genetics (ACMG), to express opposition to the then draft legislation of the Patent Eligibility Restoration Act (PERA). To gain further insight, AMP purposefully included the staff of the bill sponsors as well. However, two weeks later, on June 22nd, Senator Chris Coons (D-DE) and Tom Tillis (R-NC) formally introduced the bill, (S. 4734), in the U.S. Senate. There is no U.S. House version. As currently written, the bill contains language that would allow DNA isolated from the body to be patented, along with “laws of nature” (i.e. the law of gravity), “natural phenomena” and “abstract ideas”. This would significantly reform Section 101 of the U.S. Patent Act, which covers patent eligibility criteria, and abrogate 150 years of case law, including AMP v. Myriad AMP continues to strongly advocate against efforts on Capitol Hill that seek to overturn the Supreme Court decision and AMP Advocacy Day 2023 was dedicated to raise awareness and educate lawmakers on the dangers posed by the PERA.
On July 27th AMP hosted 70 virtual congressional meetings to discuss the impacts the bill would have on patient care and innovation. In August, Senators Tillis and Coons expressed interest in having a hearing for PERA and holding stakeholder roundtable discussions. AMP will continue to monitor developments, though it is highly unlikely this bill will move forward.
AMP Convenes Diverse Stakeholder Group to Discuss the Economic Impact of Molecular Diagnostic Testing
On September 13th AMP held the third Molecular Pathology Economics Summit. AMP believes it is important that the rapid and continuously evolving field of molecular diagnostics are simultaneously supported by systems that are equipped to change and evolve with it. As such, AMP created a platform for diverse stakeholders to discuss current challenges in the field that hinder patient access to molecular diagnostics. This day-long Economics Summit was attended by 60 individuals representing various organizations including diagnostic and pharmaceutical companies, trade associations, professional organizations, laboratories, and patient advocacy groups.
During the morning session, a series of roundtable discussions were held, each focused on a specific stakeholder perspective and their approach to the unique challenges they currently face in the realm of molecular diagnostic coding, coverage, and reimbursement. These interactive, candid discussions explored barriers to patient access, as well potential solutions and/or novel approaches to overcoming these barriers. Furthermore, AMP held breakout sessions in the afternoon to identify shared policy priorities. Stakeholders worked together to determine practical and applicable solutions. AMP was pleased to facilitate this event and foster a collaborative community of highly engaged participants that are committed to implementing this shared vision.
More information regarding the summit can be found on AMP’s 2023 Economic Summit Webpage. AMP will keep you informed of any developments and will share the AMP 2023 Economics Summit Summary of Themes once finalized. A special thank you to AMP's Corporate Advisory Council and Telcor for their generous support of the event.
.png)
Lunch and Learn: Patient Resources
Where We are Now, Where We Are Going
In June 2023, AMP hosted a virtual Lunch and Learn event on our patient facing materials titled: “Patient Resources Where We Are Now, and Where We are Going”. The Lunch and Learn featured Dr. Jill Murrell, the Chair of the PRC Patient Engagement Subcommittee, along with Maria Alejandra Diaz-Miranda, PhD. Dr. Diaz-Miranda was integral in the release of our first Spanish translated graphic “Inside a Molecular Diagnostic Laboratory/Una Mirada a Un Laboratorio Clinico de Diagnostico Molecular” in 2022. This event had 10 attendees from various patient advocate groups who are all invested in the molecular pathology space. Dr. Jill Murrell discussed the various projects of the Patient Engagement Subcommittee, including, various infographics, a new patient outreach website, and regularly hosting Lunch and Learns to directly connect patients to molecular pathologists. Dr. Diaz-Miranda then introduced AMP’s first Spanish translated Graphic and the Q&A session was very engaging.

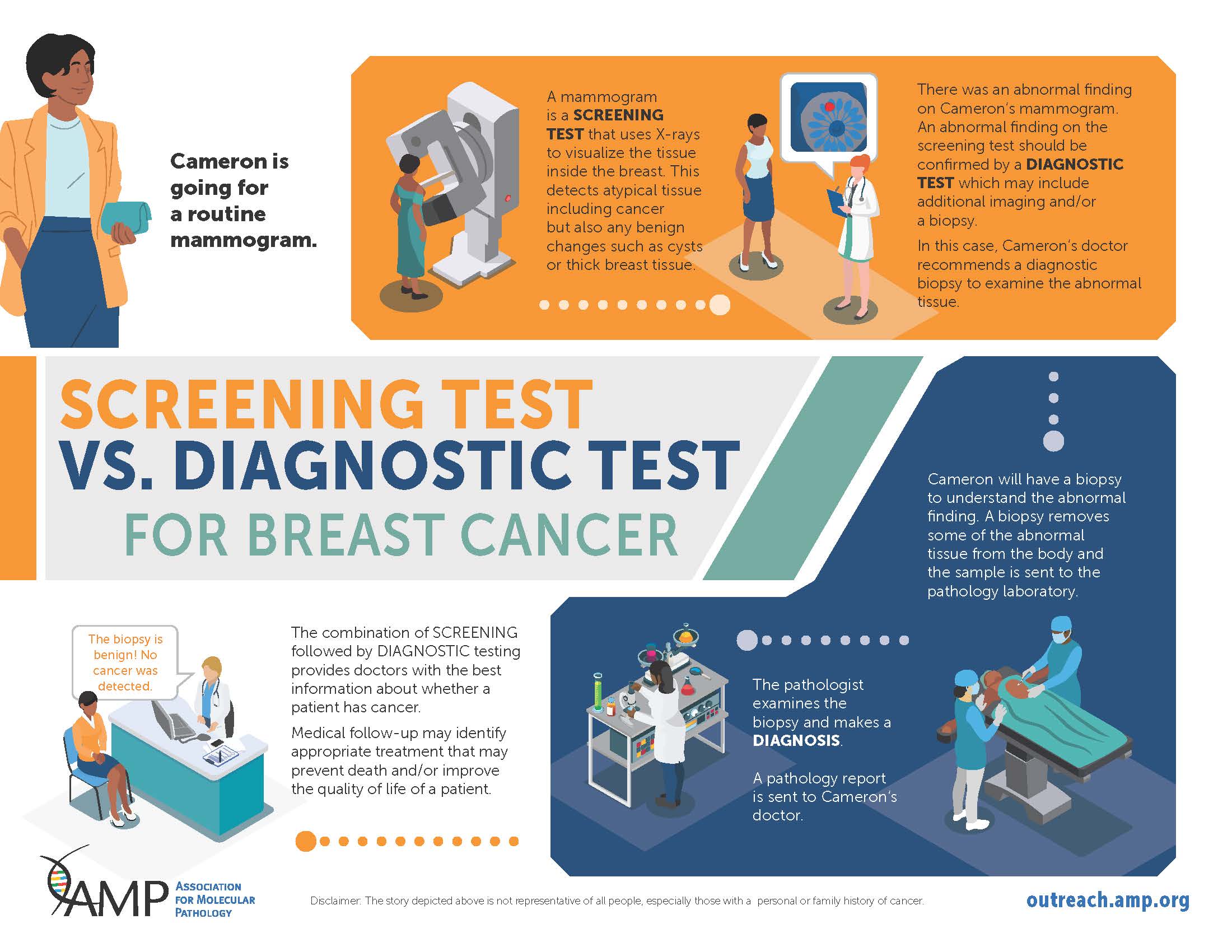
Screening vs Diagnostic Infographic Series
AMP is excited to announce an infographic series featuring the differences between Screening and Diagnostic tests. Our first graphic focuses on Newborn Screening, while the second follows a patient through their journey of Breast Cancer Screening. Currently, the PRC Patient Engagement Subcommittee has chosen to highlight Hepatitis C as the final installment of this series. Check out these resources and more on our Patient Advocacy at https://outreach.amp.org/.
AMP comments incorporated in the National Biodefense Science Board (NBSB) recommendations to HHS secretary
In August, AMP provided verbal comments to the National Biodefense Science Board (NBSB). The NBSB provides expert advice and guidance to the Secretary of HHS and the Assistant Secretary for Preparedness and Response (ASPR) on scientific, technical, and other matters related to public health emergency preparedness and response. The Board held a public meeting based on lessons learned from the COVID-19 pandemic to improve the operational public health and health system data for disaster response. The second recommendation of the Board focused on diagnostics and the draft language narrowly called for authorization to adapt tests developed by the World Health Organization (WHO) or other internationally recognized organizations if domestic tests are not available. AMP requested the Board to include language based on the PRC’s previously developed position, and the formal recommendation of the NBSB incorporated “laboratory developed testing procedures” and the avoidance of duplicative regulatory requirements during a public health emergency (PHE) in their finalized report, which can be found here. You can also view additional information on the NBSB meeting page. The recommendations are currently being reviewed by HHS leadership to inform responses to future emergencies.

Recent Comment Letters:
AMP Comments on House Ways and Means Committee RFI on ways to address chronic disparities in access to health care in rural and underserved communities - October 5, 2023
AMP Statement on FDA Proposed Rulemaking - October 3, 2023
AMP response to NGS on Molecular Pathology Procedures AMP Comments on Genetic Testing for Oncology Novitas and First Coast ( DL39367, DL39365) - September 8, 2023
AMP Comments to the Senate on the Pandemic and All-Hazards Preparedness Act (PAHPA) - July 11, 2023
AMP Comments on House Energy and Commerce Hearing on the Pandemic and All-Hazards Preparedness Act (PAHPA) - June 12, 2023
AMP sign on in Support of SALSA - May 25, 2023
The AMP EAC and PRC work diligently to provide input to Congress and relevant agencies on all issues affecting regulation and reimbursement of molecular procedures. You can peruse all recent comment letters here.






















